Chicago, Illinois – October 14, 2015 (BUSINESS WIRE) – joimax®, the global German developer and marketer of technologies and training methods for minimally invasive endoscopic spinal surgery, launches its two newest products – the EndoLIF® On-Cage and iLESSYS® Delta interlaminar system, during the 30th annual North American Spine Society (NASS) meeting in Chicago.
“With the new EndoLIF® program, combined with the TESSYS® technology, and now the iLESSYS® Delta, joimax® offers complete endoscopic solutions for spinal decompression, stabilization and fusion.”
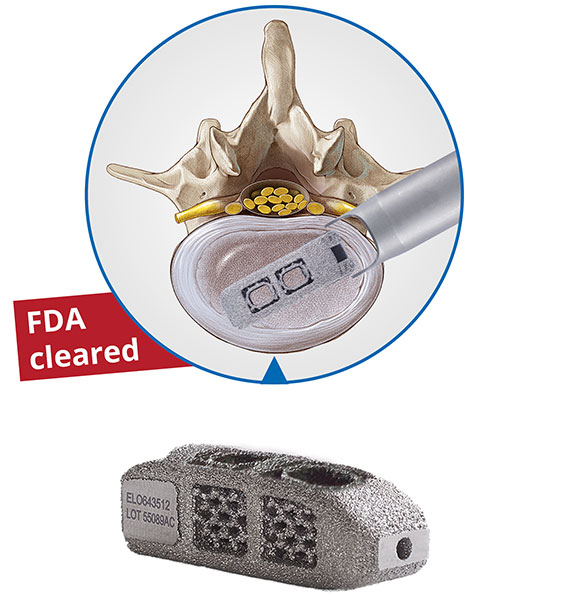
The EndoLIF® On-Cage is a 3D-printed titanium alloy, manufactured utilizing a special electron beam melting (EBM) technology. The cage displays a porous surface with a diamond cell structure, which provides an optimal base for cell proliferation and bone growth. Two large implant graft windows, which are filled with autogenous bone, allow for bridging bone to further support fusion. The EndoLIF® On-Cage implant allows surgeons to utilize a mini-open or an inter-muscular approach, similar to a mini-transforaminal lumbar interbody fusion (TLIF) approach, into the intervertebral disc space facilitating endoscopic-assisted fusion.
Dr. Ralf Wagner, LIGAMENTA Spine Center, Frankfurt, and Dr. Bernd Illerhaus, ONZ Spine Center, Recklinghausen, are two German spine specialists who have already performed more than 200 of the 800 EndoLIF® On-Cage procedures in Europe. “The access is dura and nerve-gentle, preserves better dorsal bony structures and limits scar tissue formation because of the stepwise tissue dilation,” said Dr. Wagner.
iLESSYS® Delta, an advanced development of the joimax iLESSYS® standard technology, is suitable for dorsal and dorso-lateral treatment of central spinal canal stenosis. The iLESSYS® Delta system enables a large area to be decompressed by means of a more widened interlaminar approach, guided by a full endoscopic direct visualization technique. The new and specific instrument set includes specially developed tools that permit an extensive, yet gentle, tissue-sparing decompression. A new endoscope with a 6 mm working channel allows the use of large shaver blades designed for bone and tissue resection, along with a range of instruments to be applied under full endoscopic view.
“We are impressed by the new endoscope which delivers outstanding image quality. With the specialized instrument set, it is finally possible to achieve decompression of central spinal canal stenosis,” said Albert E. Telfeian, M.D., Ph.D., Department of Neurosurgery, Rhode Island Hospital, Providence, RI. Jian Shen, MD, PhD, of Mohawk Valley Orthopedics, P.C., NY, concurs. The two surgeons gained experience with the new system during a meeting at the Anatomy Department of the University of Mainz, Germany, earlier this year, and during the cadaveric training course at Vitruvian lab in Baltimore, MD, held last week.
“Albert Telfeian and Jian Shen will be the first spinal surgeons in the USA to perform surgeries with the new iLESSYS® Delta endoscopic technology and the EndoLIF® On-Cage implants,” says Wolfgang Ries, CEO and founder of joimax®. He adds, “With the new EndoLIF® program, combined with the TESSYS® technology, and now the iLESSYS® Delta, joimax® offers complete endoscopic solutions for spinal decompression, stabilization and fusion.”
With the FDA approval of Percusys® percutaneous screw-rod-system earlier this year, and the FDA approval of EndoLIF® On-Cage in July, joimax® is positioning itself as a clear leader in minimally invasive endoscopic spinal surgery, providing a full spectrum of product technologies, now fully available in the U.S. market.