FDA Clearance for joimax® Vaporflex® and Legato® electrosurgical probes
Irvine, CA and Karlsruhe, Germany – November 9th, 2016 – joimax®, the global acting German developer and marketer of technologies and training methods for endoscopic minimally-invasive spinal surgery, today announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its Vaporflex® and Legato® electrosurgical probes with radiowave technology for open and endoscopic spine surgery.
Those joimax® Electrosurgical Instruments are comprised of a series of instruments to facilitate the delivery of electrical energy from an RF generator to the patient tissue for use in cutting and/or coagulation of tissue. All Vaporflex® and Legato® probes use radio frequency which is generated by high oscillating electrical current received from a commercially available RF generator.
The new Vaporflex® system is optimized for versatile use due to different lengths, diameters and probe tips to allow endoscopic transforaminal and interlaminar procedures. It is both compatible and cleared also for the use with all Endovapor®/SurgiMax® and SurgiMax® Plus electrosurgical units and enables an easy swap of the Triggerflex® to the new Vaporflex® system. The bipolar Vaporflex® probes can easily be adopted to the complete range of joimax® systems. joimax® offers all its probes in a kit containing a sterilization tray, the hand piece as well as a shaft and a connection cable.
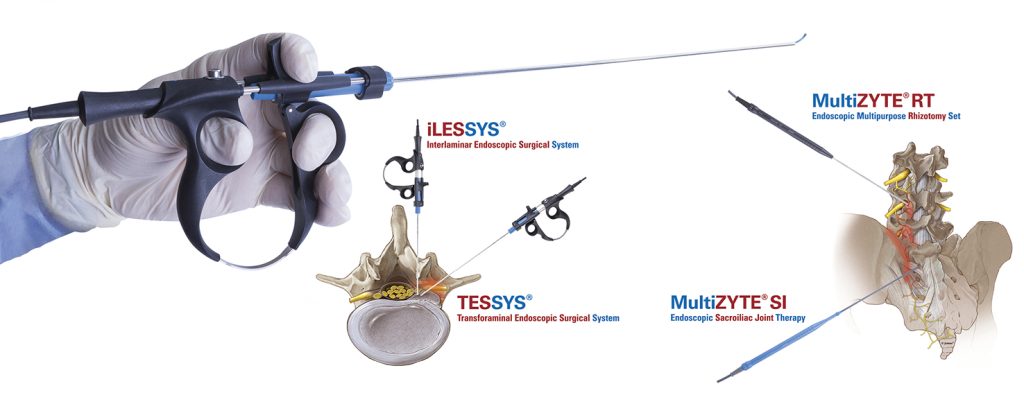
The monopolar and bipolar Legato® probes which support the rhizotomy procedures with the existing Multiuse and both newly launched products MultiZYTE® RT for Facet Joint Denervation and MultiZYTE® SI for SI Joint Therapy.
“Both the Vaporflex® and Legato® probes are ready for several generations of radiofrequency generators” says Wolfgang Ries, CEO and founder of joimax®. “Spinal surgeons are now enabled to perform the range of joimax® procedures with even more flexibility and ergonomics in the USA as well. This way we further enhance surgical interventions in endoscopic minimally-invasive spinal surgery” he continues.
About joimax®
Founded in Karlsruhe, Germany in 2001, joimax® is the leading developer and marketer of complete systems for full-endoscopic and minimally invasive spinal surgery. With the Endoscopic Surgical Systms TESSYS® (transforaminal), iLESSYS® (interlaminar) and CESSYS® (cervical) for decompression procedures, MultiZYTE® for facet and sacroiliac joint pain treatment, EndoLIF® and Percusys® for minimally invasive endoscopically assisted stabilizations, established systems are provided, addressing a whole range of indications. All methods are supported by the latest generation electronical devices in the all new NAVENTO® navigated endoscopic tower. In procedures for herniated discs, stenosis, pain therapy or spinal stabilization treatment, surgeons utilize joimax® technologies to operate through small incisions under local or full anesthesia, via tissue and muscle-sparing corridors, and through natural openings in the spinal canal, e.g., the intervertebral foramen, the so-called "Kambin triangle".
Contacts
Press Contact Germany
joimax® GmbH
Antje Paulsen
antje.paulsen@joimax.com
+49-721-25514-214
Press Contact USA
joimax®, Inc.
Katherine Ray
Katherine.Ray@joimaxusa.com
+1 949 859 3472 ext. 301